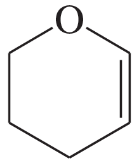
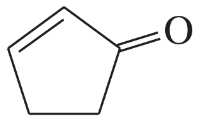
Circle the functional groups in the following structures. State to which class (or classes) of compounds the structure belongs.
(g)
(h)
(i)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 1:49m
1:49mMaster Why we need functional groups. with a bite sized video explanation from Johnny
Start learning