Indicate the number of signals and the multiplicity of each signal in the 1H NMR spectrum of each of the following compounds:
c. ClCH2CH2CH2Cl
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: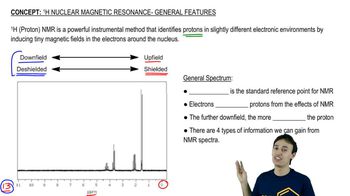
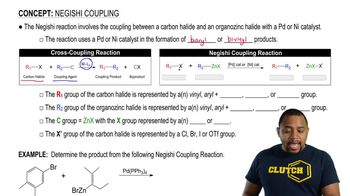
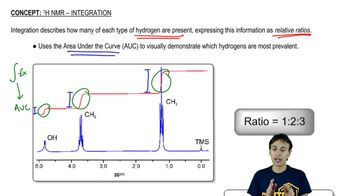

 12:21m
12:21mMaster Splitting without J-values with a bite sized video explanation from Johnny
Start learning