Multiple Choice
What is the oxidation state of the carbonyl carbon in the following molecule?
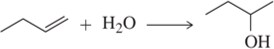
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: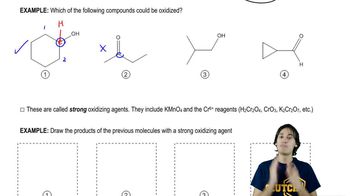
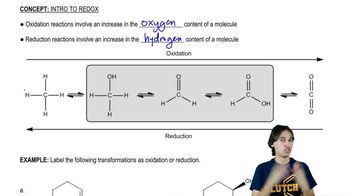


 6:02m
6:02mMaster General Features of Redox with a bite sized video explanation from Johnny
Start learning