Show how you would accomplish the following syntheses efficiently (you may use any necessary reagents).
(a) trans-1-bromobut-2-ene → trans-pent-3-enoic acid (two ways)
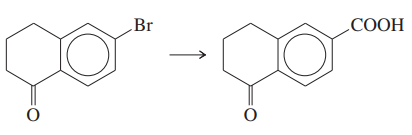
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: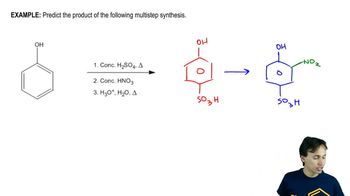


 2:12m
2:12mMaster Carbonation of Grignard Reagents with a bite sized video explanation from Johnny
Start learning