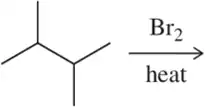
When (1R,3S)-1-tert-butyl-1,3-dimethylcyclopentane is halogenated, one stereoisomer is produced in excess.
(b) explain why this reaction did not produce an equal mixture of stereoisomers.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 2:05m
2:05mMaster The one reaction that alkanes will actually undergo. with a bite sized video explanation from Johnny
Start learning