Multiple Choice
Use FMOT to predict the mechanism and products for the following cycloaddition. If no product is favored, write “symmetry-disallowed” in place of the product.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: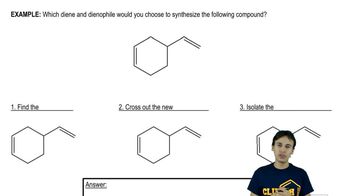
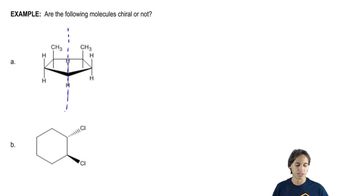

 9:36m
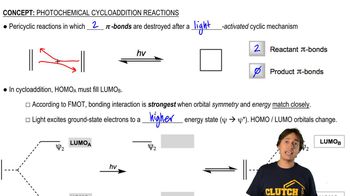
9:36mMaster MO Theory of Photochemical Cycloadditions with a bite sized video explanation from Johnny
Start learning