Open Question
Use the cycloaddition summary rules to verify that you have come to the correct conclusion.
 Verified step by step guidance
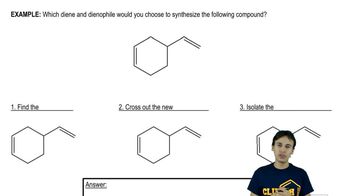
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: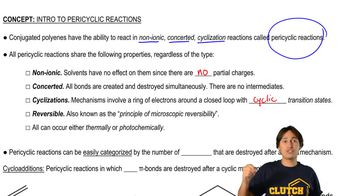


 9:36m
9:36mMaster MO Theory of Photochemical Cycloadditions with a bite sized video explanation from Johnny
Start learning