Predict the products of the following reactions. An excess of acid is available in each case.
(c) anisole (methoxybenzene) + HBr
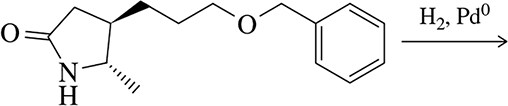
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 4:22m
4:22mMaster How to predict the products of Ether Cleavage. with a bite sized video explanation from Johnny
Start learning