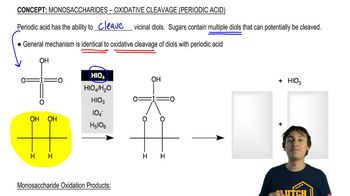
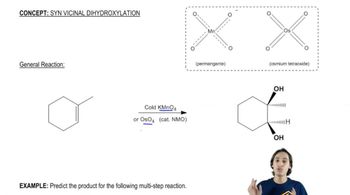
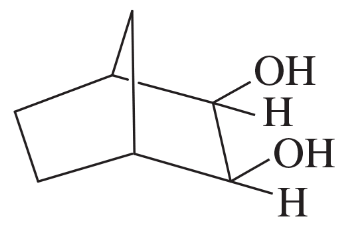
What product is obtained from the reaction of each of the following alcohols with
c. the regents required for a Swern oxidation?
1. 3-pentanol
2. 1-pentanol
3. 2-methyl-2-pentanol
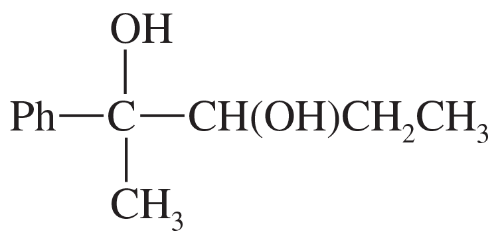
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: