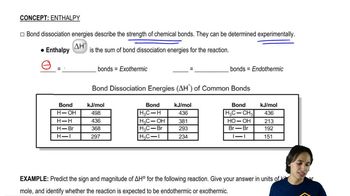
A student adds NBS to a solution of 1-methylcyclohexene and irradiates the mixture with a sunlamp until all the NBS has reacted. After a careful distillation, the product mixture contains two major products of formula C7H11Br.
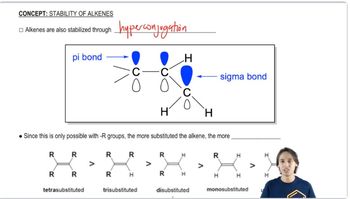
b. Rank these three intermediates from most stable to least stable.