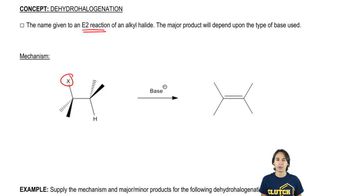
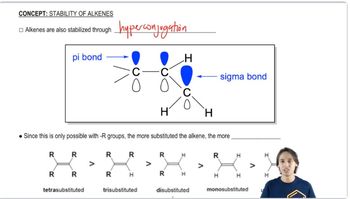
Predict the products formed by sodium hydroxide-promoted dehydrohalogenation of the following compounds. In each case, predict which will be the major product.
a. 1-bromobutane
b. 2-chlorobutane
c. 3-bromopentane
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 3:02m
3:02mMaster The dehydrohalogenation mechanism. with a bite sized video explanation from Johnny
Start learning