Multiple Choice
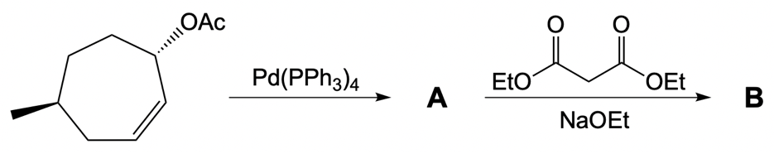
Determine the allylic halide and enolate used to create the following product via a catalytic allylic alkylation reaction.
 Verified step by step guidance
Verified step by step guidance
 3:21m
3:21mMaster Catalytic Allylic Alkylation Concept 1 with a bite sized video explanation from Johnny
Start learning
