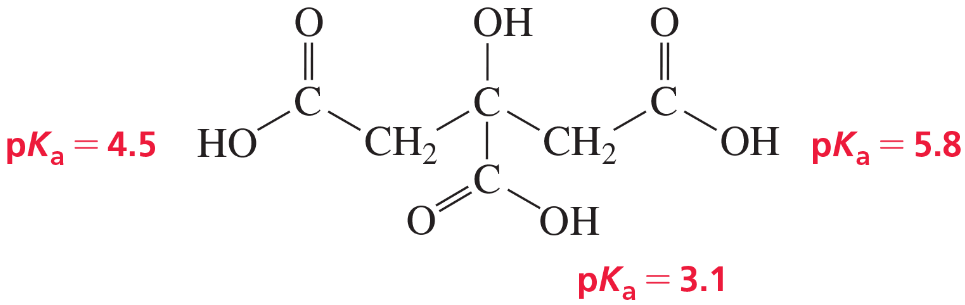
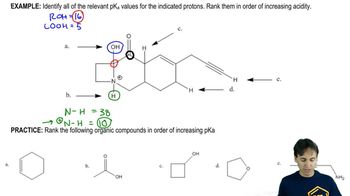
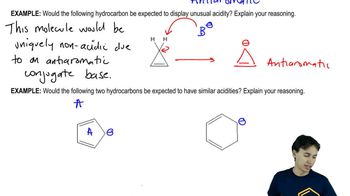
a. Which is a stronger base: CH3COO− or HCOO−? (The pKa of CH3COOH is 4.8; the pKa of HCOOH is 3.8.)
b. Which is a stronger base: HO− or -NH2? (The pKa of H2O is 15.7; the pKa of NH3 is 36.)
c. Which is a stronger base: H2O or CH3OH? (The pKa of H3O+ is −1.7; the pKa of CH3O+H2 is −2.5.)