For each compound, indicate the atom that is most apt to be protonated.
a.
b.
c.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: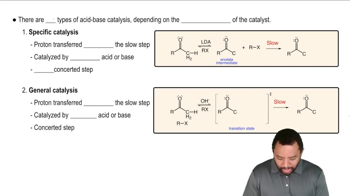

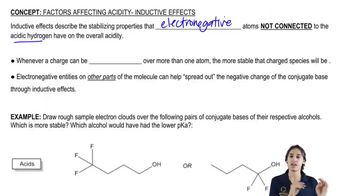

 9:36m
9:36mMaster The 12 pKa values you want to memorize (because they are important!). with a bite sized video explanation from Johnny
Start learning