Within each set of structures, indicate which will react fastest, and which slowest, toward nucleophilic addition in basic conditions.
(b)
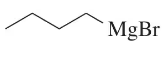
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 8:27m
8:27mMaster Nucleophilic Addition with a bite sized video explanation from Johnny
Start learning