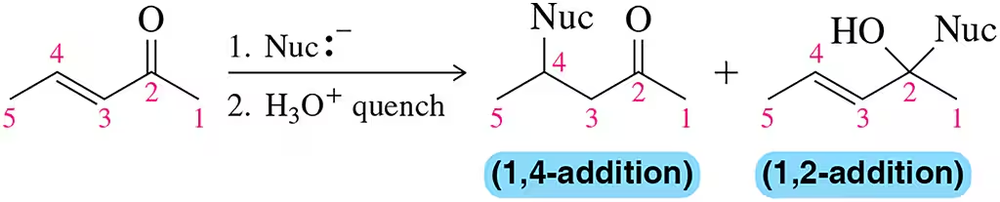
Classify the following nucleophiles as strong, weak, or intermediate. Would you expect each to add to a carbonyl directly or wait for a carbocation to form?
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 8:27m
8:27mMaster Nucleophilic Addition with a bite sized video explanation from Johnny
Start learning