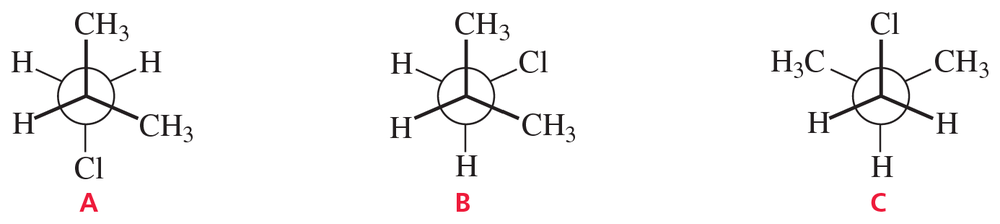
Which of the following structures represent the same compound? Which ones represent different compounds?
(f)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:11m
3:11mMaster How sigma bond rotation is visualized with a bite sized video explanation from Johnny
Start learning