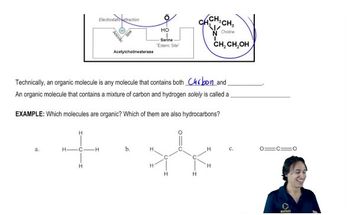
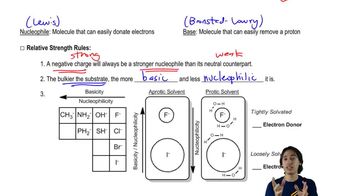
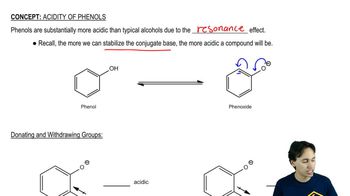
Rank each set of compounds in order of increasing boiling points.
(a) triethylamine, di-n-propylamine, n-propyl ether
(b) ethanol, dimethylamine, dimethyl ether
(c) diethylamine, diisopropylamine, trimethylamine
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: