For each of the following processes, indicate whether you expect ∆S° to be greater than, less than, or equal to 0. Explain your answer.
(c)
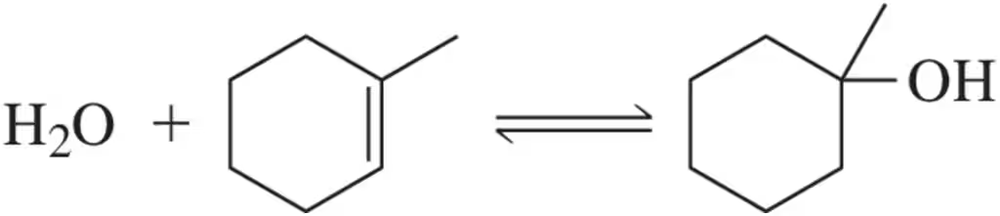
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:46m
2:46mMaster Explaining what entropy is. with a bite sized video explanation from Johnny
Start learning