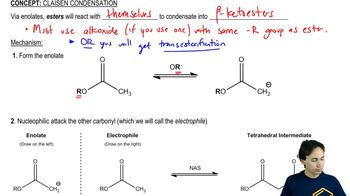
Predict the products from crossed Claisen condensation of the following pairs of esters. Indicate which combinations are poor choices for crossed Claisen condensations.
(c)
(d)
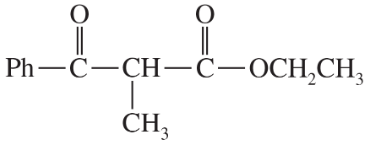
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: