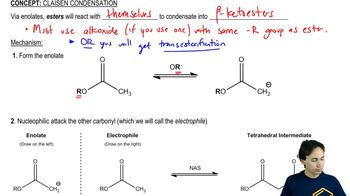
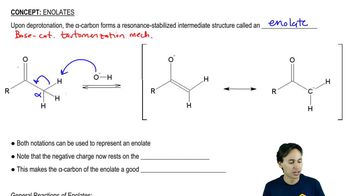
Ethoxide is used as the base in the condensation of ethyl acetate to avoid some unwanted side reactions. Show what side reactions would occur if the following bases were used.
(a) sodium methoxide
(b) sodium hydroxide
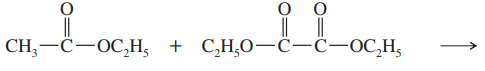
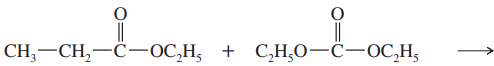
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: