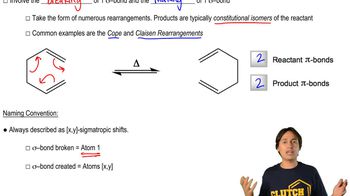
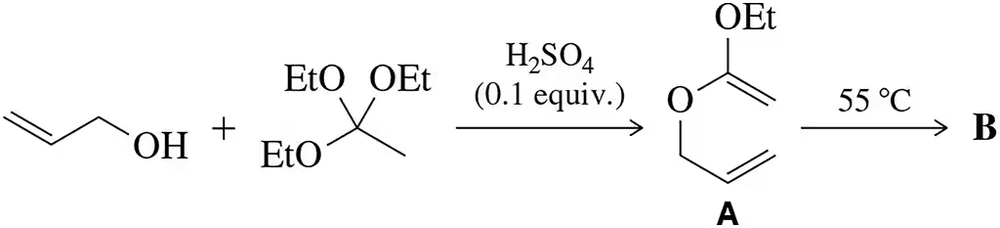The Claisen rearrangement can be used to attach allyl groups to benzene via initial alkylation of a phenol.
(a) Predict the identity of A and B
(b) provide an arrow-pushing mechanism for each step. [The phenol alkylation is simply a Williamson ether synthesis reaction.]