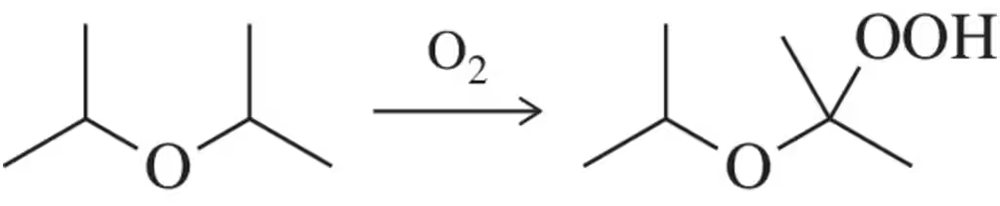
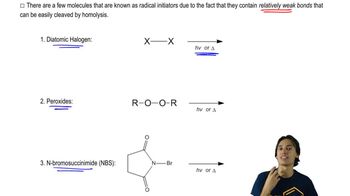
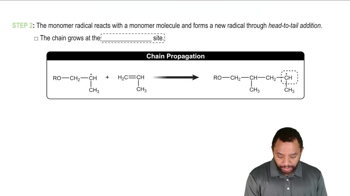
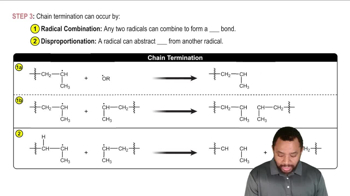
Free-radical bromination of the following compound introduces bromine primarily at the benzylic position next to the aromatic ring. If the reaction stops at the monobromination stage, two stereoisomers result.
a. Propose a mechanism to show why free-radical halogenation occurs almost exclusively at the benzylic position.
b. Draw the two stereoisomers that result from monobromination at the benzylic position.
c. Assign R and S configurations to the asymmetric carbon atoms in the products.