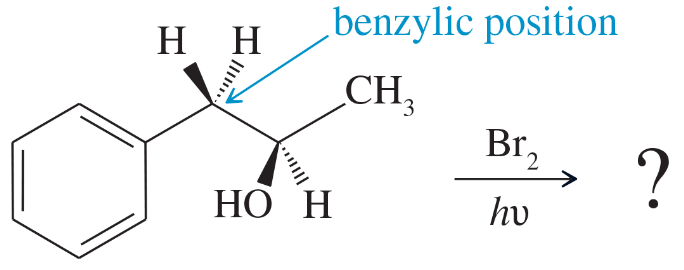
For each alkane,
2. determine whether free-radical chlorination would be a good way to make any of these monochlorinated derivatives. Will the reaction give mostly one major product?
a. Cyclopentane
b. Methylcyclopentane
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: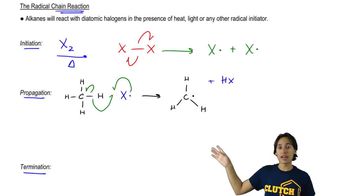

 2:05m
2:05mMaster The one reaction that alkanes will actually undergo. with a bite sized video explanation from Johnny
Start learning