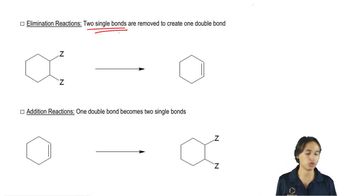
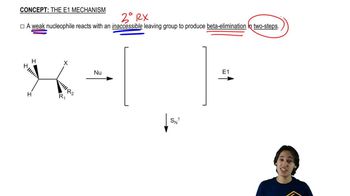
Pure (S)-2-bromo-2-fluorobutane reacts with methoxide ion in methanol to give a mixture of (S)-2-fluoro-2-methoxybutane and three fluoroalkenes.
b. Propose a mechanism to show how (S)-2-bromo-2-fluorobutane reacts to give (S)-2-fluoro-2-methoxybutane. Has this reaction gone with retention or inversion of configuration?