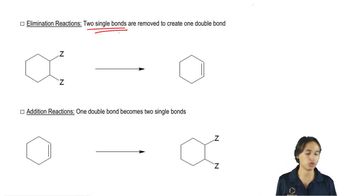
Pure (S)-2-bromo-2-fluorobutane reacts with methoxide ion in methanol to give a mixture of (S)-2-fluoro-2-methoxybutane and three fluoroalkenes.
a. Use mechanisms to show which three fluoroalkenes are formed.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: