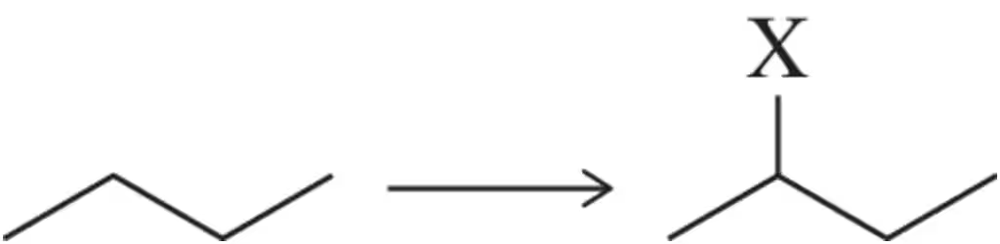
Suppose you intend to synthesize the secondary alkyl halide from each reaction shown.
(a) What is the atom economy of each reaction?
(c) Which reaction would you consider greener? Why?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 4:32m
4:32mMaster Explaining relative rates of halogenation. with a bite sized video explanation from Johnny
Start learning