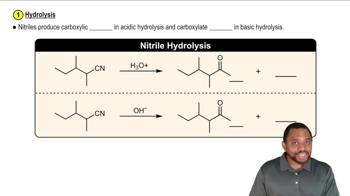
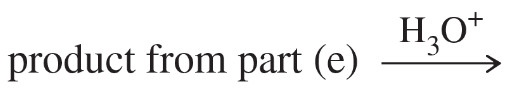α-Amino acids can be prepared by treating an aldehyde with ammonia/trace acid, followed by hydrogen cyanide, followed by acid-catalyzed hydrolysis.
b. What amino acid is formed when the aldehyde that is used is 3-methylbutanal?
c. What aldehyde is needed to prepare isoleucine?