Show how you would accomplish the following syntheses. Some of these conversions may require more than one step.
(e) cyclohexylamine → N-cyclohexylacetamide
(f) bromocyclohexane → dicyclohexylmethanol

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: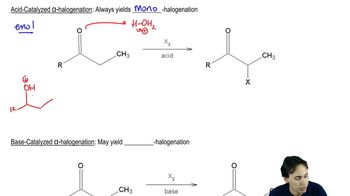


 9:32m
9:32mMaster NAS - The Three Rules with a bite sized video explanation from Johnny
Start learning