Show how you would synthesize the following esters from appropriate acyl chlorides and alcohols.
(e) tert-butyl acetate
(f) diallyl succinate
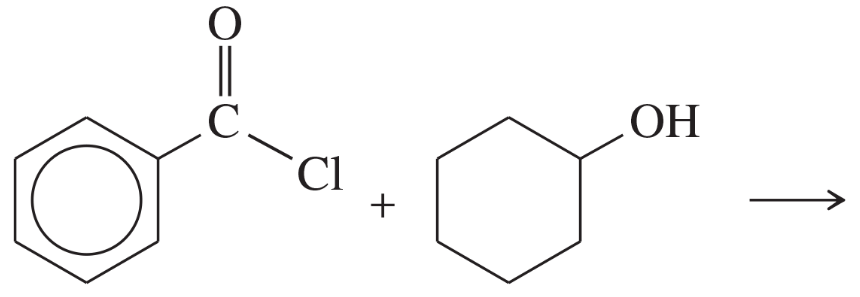
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 9:32m
9:32mMaster NAS - The Three Rules with a bite sized video explanation from Johnny
Start learning