Textbook Question
Pentane-2,4-dione (acetylacetone) exists as a tautomeric mixture of 8% keto and 92% enol forms. Draw the stable enol tautomer, and explain its unusual stability.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: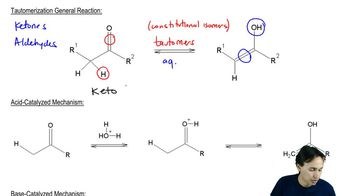


 4:48m
4:48mMaster Tautomers of Dicarbonyls with a bite sized video explanation from Johnny
Start learning