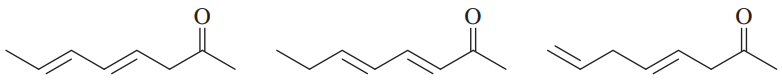
Of the compounds you named in Problem 45:
b. Which is the least stable?
a.
b.
c.
d.
e.
f.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
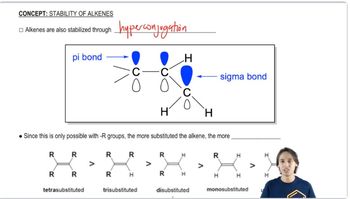

 4:28m
4:28mMaster Understanding trends of alkene stability. with a bite sized video explanation from Johnny
Start learning