For each set of isomers, choose the isomer that you expect to be most stable and the isomer you expect to be least stable.
(b)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: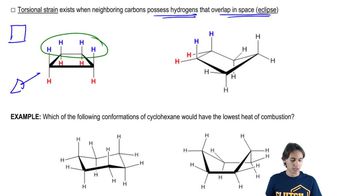
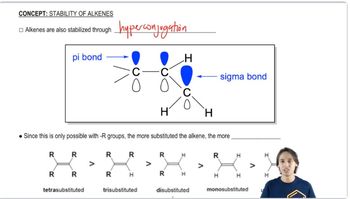

 4:28m
4:28mMaster Understanding trends of alkene stability. with a bite sized video explanation from Johnny
Start learning