Show how each of the following compounds could be prepared from the given starting material. Each requires a protecting group.
a.
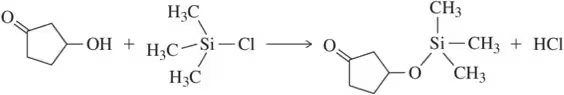
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 7:16m
7:16mMaster Mechanism of Silyl Ether Protecting Groups. with a bite sized video explanation from Johnny
Start learning