Suggest a synthesis for the following molecules beginning with organic molecules containing three or fewer carbons. [You will need to use a protecting group in these syntheses.]
(c)
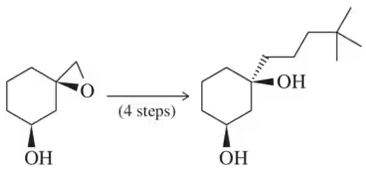
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: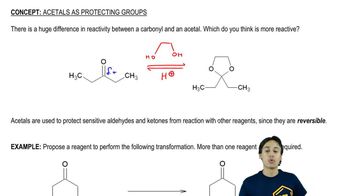

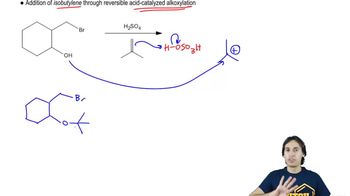

 7:16m
7:16mMaster Mechanism of Silyl Ether Protecting Groups. with a bite sized video explanation from Johnny
Start learning