Predict which member of each pair is more soluble in water. Explain your prediction.
(e)
(f)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
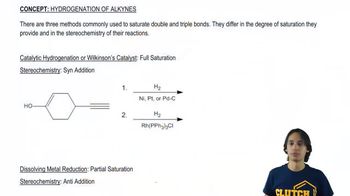

 1:47m
1:47mMaster Understanding “like dissolves like”. with a bite sized video explanation from Johnny
Start learning