Circle the member of each pair that is more soluble in water.
a. CH3CH2OCH2CH3 or CH3CH2CH2CH2CH3
b. CH3CH2OCH2CH3 or CH3CH2CH2OH
c. CH3CH2NHCH3 or CH3CH2CH2CH3
d. CH3CH2OH or CH3CH2CH2CH2OH
e.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: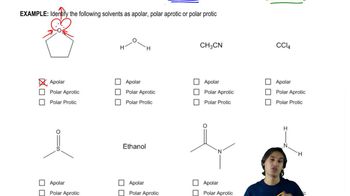


 1:47m
1:47mMaster Understanding “like dissolves like”. with a bite sized video explanation from Johnny
Start learning