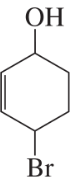
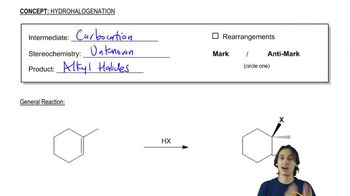
When Br2 is added to buta-1,3-diene at –15 °C, the product mixture contains 60% of product A and 40% of product B. When the same reaction takes place at 60 °C, the product ratio is 10% A and 90% B.
d. If you had a solution of pure A, and its temperature were raised to 60 °C, what would you expect to happen? Propose a mechanism to support your prediction.