Textbook Question
The heat of combustion of cis-1,2-dimethylcyclopropane is larger than that of the trans isomer. Which isomer is more stable? Use drawings to explain this difference in stability.
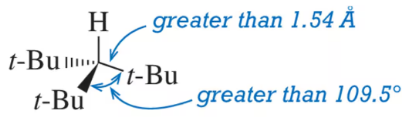
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:11m
1:11mMaster Understanding Heat of Combustion with a bite sized video explanation from Johnny
Start learning