Which of the following pairs of compounds could be separated by recrystallization or distillation?
(c)
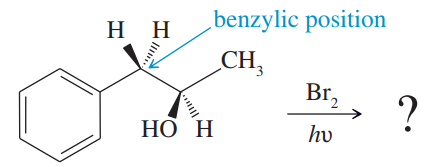
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:44m
3:44mMaster Using chiral centers to predict types of stereoisomers. with a bite sized video explanation from Johnny
Start learning