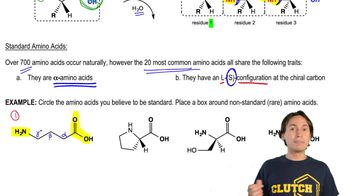
A decapeptide undergoes partial hydrolysis to give peptides whose amino acid compositions are shown. Reaction of the intact decapeptide with Edman's reagent releases PTH-Gly. What is the sequence of the decapeptide?
1. Ala, Trp
2. Val, Pro, Asp
3. Pro, Val
4. Ala, Glu
5. Trp, Ala, Arg
6. Arg, Gly
7. Glu, Ala, Leu
8. Met, Pro, Leu, Glu