Multiple Choice
For a 2nd order substitution reaction tripling the concentration of the substrate, R–X, and tripling the concentration of the nucleophile does what to the rate of the reaction?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
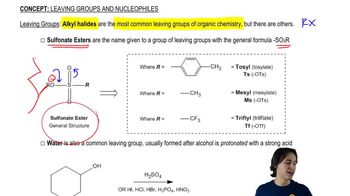


 8:33m
8:33mMaster Drawing the SN2 Mechanism with a bite sized video explanation from Johnny
Start learning