Textbook Question
What is each compound's systematic name?
g.
h.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: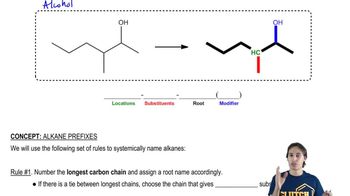


 6:42m
6:42mMaster Understanding Non-IUPAC Substituents with a bite sized video explanation from Johnny
Start learning