Draw the pH–activity profile for an enzyme that has one catalytic group at the active site:
a. the catalytic group is a general-acid catalyst with a pKa = 5.6.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 4:41m
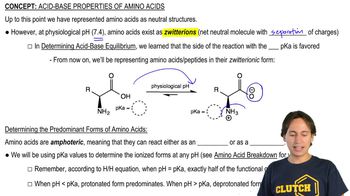
4:41mMaster Why Amino Acids Exist as Zwitterions with a bite sized video explanation from Johnny
Start learning