Which of the following pure compounds can form hydrogen bonds? Which can form hydrogen bonds with water? Which ones do you expect to be soluble in water?
e. CH3(CH2)3CH3
f. CH2=CH—CH2CH3
g. CH3COCH3
h. CH3CH2COOH
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: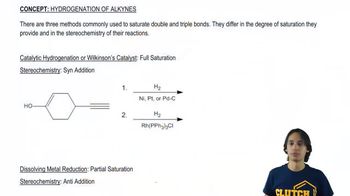



 3:08m
3:08mMaster How IMFs are related to melting and boiling points. with a bite sized video explanation from Johnny
Start learning