Predict which compound in each pair has the higher boiling point. Explain your prediction.
(a) CH3CH2OCH3 or CH3CH(OH)CH3
(b) CH3CH2CH2CH3 or CH3CH2CH2CH2CH3
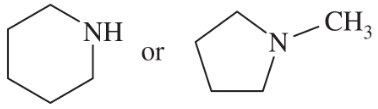
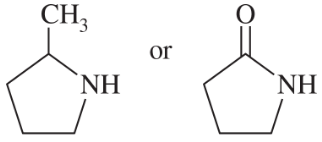
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:08m
3:08mMaster How IMFs are related to melting and boiling points. with a bite sized video explanation from Johnny
Start learning