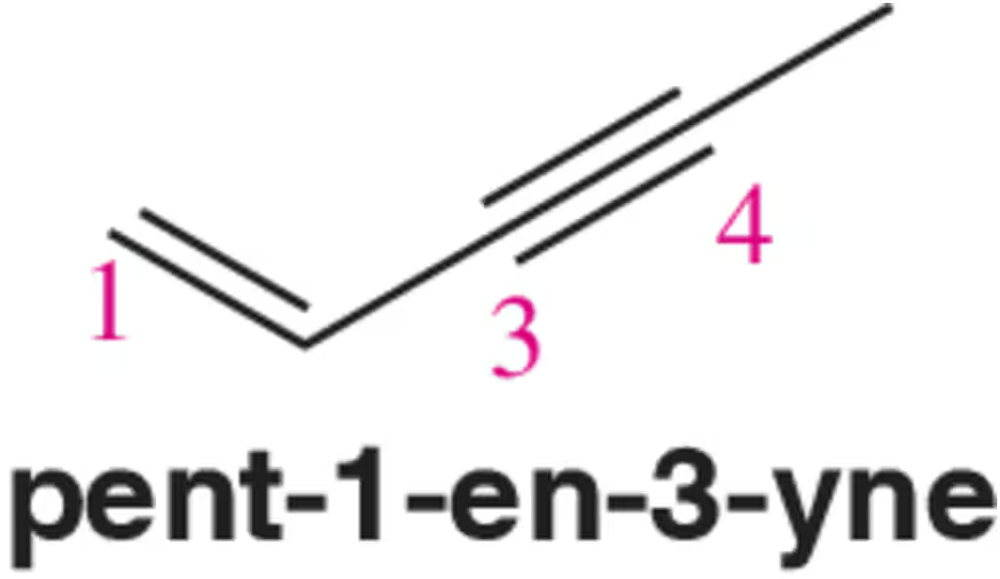
Textbook Question
In your own words, explain why it is not possible to make primary alkyl halides, such as 1-bromopentane, using the electrophilic addition of HCl or HBr to an alkene.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:38m
3:38mMaster How to add to asymmetrical double bonds. with a bite sized video explanation from Johnny
Start learning