Textbook Question
To which carbon would you expect an electrophile to add (1, 3, or 4)? Explain your answer.
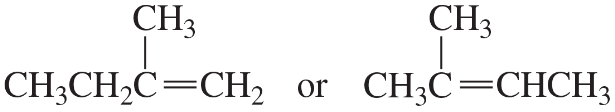
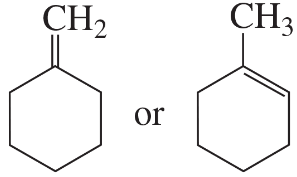
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:38m
3:38mMaster How to add to asymmetrical double bonds. with a bite sized video explanation from Johnny
Start learning