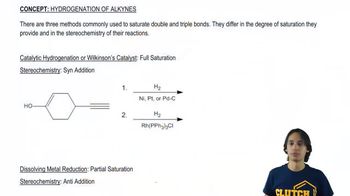
N-Methylpyrrolidine has a boiling point of 81 °C, and piperidine has a boiling point of 106 °C.
b. Tetrahydropyran has a boiling point of 88 °C, and cyclopentanol has a boiling point of 141 °C. These two isomers have a boiling point difference of 53 °C. Explain why the two oxygen-containing isomers have a much larger boiling point difference than the two amine isomers.