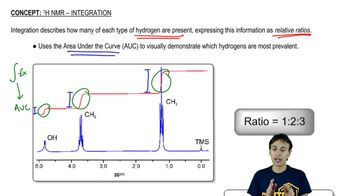
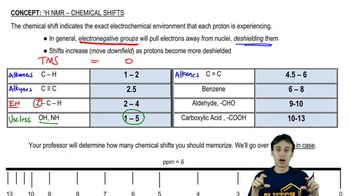
An alkyl halide reacts with an alkoxide ion to form a compound whose 1H NMR spectrum is shown here. Identify the alkyl halide and the alkoxide ion. (Hint: See Section 9.15.)
<IMAGE>
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: